Laquinimod
Laquinimod is a first-in-class immunomodulator with a novel mode of action in development for the treatment of severe inflammatory eye diseases such as non-infectious uveitis.
This is laquinimod
It has been shown in experimental models of autoimmune/inflammatory diseases that laquinimod targets the aryl hydrocarbon receptor (AhR) that is present in antigen-presenting cells and involved in the regulation of these cells. By targeting the AhR, antigen presenting cells are re-programmed to become tolerogenic, so that instead of activating pro-inflammatory T cells, regulatory T cells with anti-inflammatory properties are activated leading to a dampening of the inflammation.

Non-Infectious Uveitis
Non-infectious uveitis (NIU) is the inflammation of the uveal tract (iris, ciliary body, and choroid), but can also lead to an inflammation of nearby tissues, such as the retina, the optic nerve, and the vitreous humor, in the absence of an infectious cause. The uvea is crucial for the delivery of oxygen and nutrients to the eye tissues, and an inflammation of the uvea can cause serious tissue damage to the eye, with symptoms including general vision problems and a risk of blindness. Furthermore, floater spots in the eye, eye pain and redness, photophobia, headache, small pupils, and alteration of iris color are common symptoms.
If left untreated, uveitis can lead to severe eye problems, including blindness, cataract, glaucoma, damage to the optic nerve, and detachment of the retina. Non-infectious uveitis often occurs in connection with systemic autoimmune diseases such as sarcoidosis, multiple sclerosis and Crohn’s disease. NIU can be divided into subtypes depending on the location of the inflammation. Intermediate, posterior and panuveitis (non-anterior non-infectious uveitis, NA-NIU) are the most severe and highly recurrent forms which can cause blindness if left untreated. Laquinimod is developed as a new treatment option for non-infectious uveitis.
The market
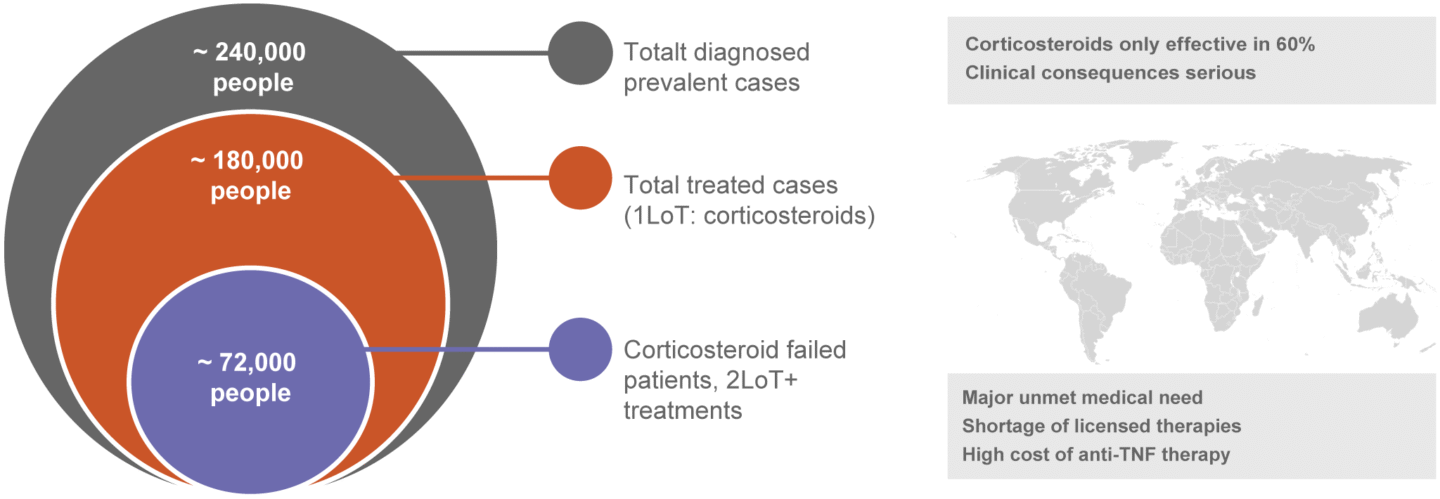
There are limited treatment options for patients with NA-NIU. The drug of choice for most patients remains long term high dose corticosteroid therapy. Still, about 40 percent of patients fail in achieving disease control, or cannot continue with high dose corticosteroids due to side effects (Rosenbaum JT. Uveitis: treatment. In: Post TW, ed. UpToDate. Waltham (MA): UpToDate; 2021).
Recently, intra-ocular corticosteroid injections have been introduced with a benefit for some patients and may limit the systemic corticosteroid-related side effects. However, the procedure of injecting a sustained release depot directly in the eye is associated with risks such as cataract and increased intraocular pressure.
Approximately 1.8 million patients in the seven major markets are expected to be diagnosed with uveitis in 2033, whereof approx. 670,000 patients are expected to received treatment. Of a total of approximately 240,000 diagnosed patients with NIU-NA, approximately 180,000 patients are expected to be treated, whereof approximately 72,000 are estimated to be refractory to corticosteroid therapy and are candidates for 2nd line therapy (Global Data Report March 2025, Uveitis – Opportunity Assessment and Forecast).
Global sales of drugs for the treatment of Uveitis amounted to approximately USD 522 million in 2023 and sales are expected to increase to approximately USD 1.5 billion by 2033 (Global Data Report March 2025, Uveitis – Opportunity Assessment and Forecast).
Uveitis: Significant opportunity
– Significant opportunity in segment of non-infectious non-anterior uveitis in 7MM, forecasts for 2033
Abbrev: LoT – Line of treatment
Source: GlobalData Report March 2025, Uveitis-opportunity Assessment and Forecast
Current treatments
The current standard treatment for patients with non-infectious uveitis is high-dose oral corticosteroids or injections of corticosteroids in or around the eye. Immunosuppressants, such as methotrexate or cyclosporin, are used as corticosteroid-sparing regimen in the 2nd line of treatment, whereas anti-TNF antibodies (Humira) are used as a 2nd or 3rd line of treatment.
There is a high unmet medical need for new effective and safe therapies for non-infectious non-anterior uveitis:
- approximately 35 percent of patients suffer from severe visual impairment with the risk of blindness
- approximately 40 percent of patients fail on corticosteroids therapy
- long-term treatment of corticosteroids in high doses is associated with severe side effects
- currently no topical treatment options are available
Therefore, there is a need for new treatments with additive effects to corticosteroids to limit failures in the 1st line of treatment. Furthermore, there is a need for safer therapies that can reduce or replace long-term use of steroids and a treatment that could be administered topically and reach to the back of the eye to minimize systemic adverse effects and to reduce injection-related risks.
Laquinimod in non-infectious uveitis
Laquinimod will be developed as a new treatment for non-infectious uveitis and has the potential to be used in the 1st line of treatment as an add-on to corticosteroids, as well as in the 2nd line of treatment for patients that have failed corticosteroid treatment.
Clinical development
An innovative eye drop formulation of laquinimod has been developed, and a preclinical safety and toxicity bridging program for topical treatment has been completed. A clinical Phase I study to document the safety and tolerability of the eye drops formulation has been performed in healthy subjects.
To ensure that laquinimod reaches the posterior chamber of the eye to support further development in patients with non-anterior uveitis, a clinical ocular biodistribution study of the eye drop formulation has been conducted.The top-line results from the LION study (Safety, Tolerability, and Distribution of Topical Laquinimod Eye Drops, an Innovative ImmunomodulatOr Targeting Aryl HydrocarboN Receptor) show that daily dose levels of either 0.6, 1.2 and 1.8 mg resulted in dose related intraocular concentrations of laquinimod, which reached a therapeutically relevant level in both the vitreous humor and anterior chamber. Laquinimod administered as eye drops at the chosen daily dose levels was safe and well tolerated for the period of administration studied, and no dose-limiting toxicities were reported in any of the subjects.
The LION study was presented at the International Ocular Inflammation Society (IOIS) Congress, the largest scientific meeting in the field of uveitis and ocular inflammation in the world, in Rio de Janeiro, Brazil, on June 26, 2025 and at the American Academy of Ophthalmology (AAO) annual meeting in October 2025.
The study has been conducted in collaboration with researchers at the Byers Eye Institute, Stanford University (Palo Alto, CA, USA) with the Principal Investigator Quan Dong Nguyen, MD, MSc, FAAO, FARVO, FASRS, Professor of Ophthalmology, Medicine, and Pediatrics, Stanford University School of Medicine.
More information about the study can be found in the box below and on www.clinicaltrials.gov.
A phase II clinical study of oral and eye drop formulations of laquinimod in patients with non-infectious uveitis is prepared. The start of the study is subjected to collaboration with a partner.
Previous clinical experience with laquinimod
During its years of advanced product development, clinical efficacy, and safety data on oral laquinimod was established in more than 5,000 patients, primarily in multiple sclerosis (MS) patients, representing more than 14,000 patient-years of exposure. Extensive datasets have also been generated, including regulatory package of preclinical and clinical safety and full commercial scale CMC documentation.
