Tasquinimod
Tasquinimod is an orally active small molecule immunomodulator with a novel mode of action, blocking tumor supporting pathways in the bone marrow microenvironment. Tasquinimod is being developed for the treatment of blood cancers, with focus on myelofibrosis.
This is tasquinimod
The tumor microenvironment in the bone marrow is essential for development of blood cancers and a key driver of disease recurrency as well as resistance to treatment.
Tasquinimod targets cells in the microenvironment of the bone marrow, immunosuppressive myeloid cells, endothelial cells, and mesenchymal cells, which play a central role in the development of blood cancers. Tasquinimod affects the function of these cells, leading to reduced tumor growth, reduced fibrosis, and restored hematopoiesis.
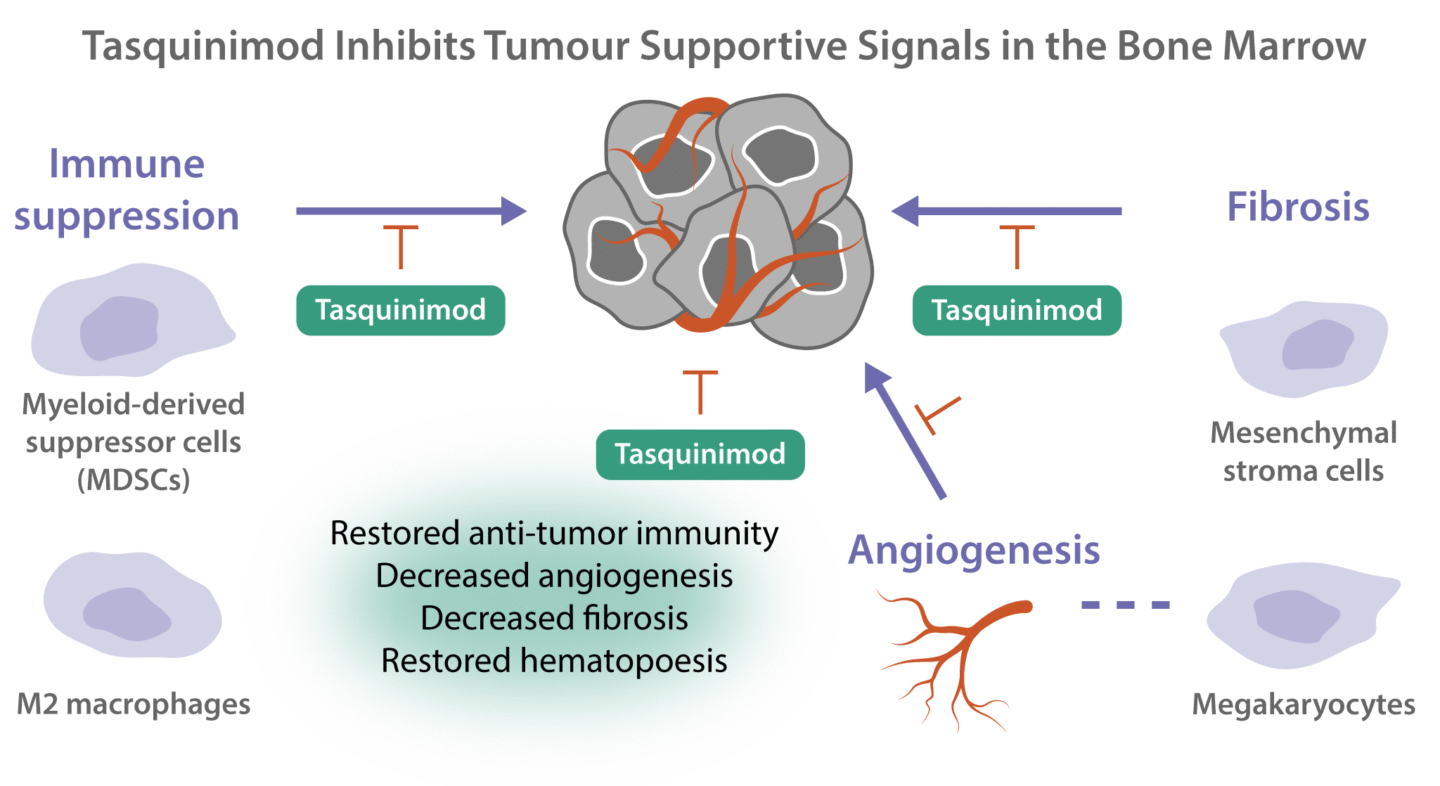
Myelofibrosis
Myelofibrosis is a rare form of blood cancer. The sex- and age-adjusted incidence is estimated at approximately 1.5 cases per 100,000 people, with a prevalence of 12 patients per 100,000 people (Slowley et al., 2024). This would translate to a prevalence of more than 100,000 people with myelofibrosis in the EU, US, UK, and Japan.
The underlying cause of myelofibrosis is unknown. Patients with myelofibrosis have an abnormal production of blood-forming cells leading to the replacement of healthy bone marrow with scar tissue (fibrosis).
Due to the lack of normal blood cell production, patients typically show laboratory value abnormalities, such as anemia and changes in white blood cell counts, and blood cell-differentiation. Later symptoms include enlargement of the spleen, an increased risk for infections, night sweats and fever. Myelofibrosis is associated with shortened survival, due to for instance bone marrow failure and transformation into acute leukemia.
Current treatments and market
Myelofibrosis can be treated with bone marrow transplantation for eligible individuals, erythropoietin to manage anemia and JAK2 inhibitors to reduce spleen size. Today the following drugs are approved for these patients as symptom-directed therapy: Hydroxy-urea, ruxolitinib, momelotinib, fedratinib and pacritinib (the latter four are JAK2 inhibitors, JAKi). At present there are no approved treatment options that would reverse bone marrow fibrosis in myelofibrosis, and there are only limited treatment options available for myelofibrosis patients whose disease progress during JAKi treatment or cannot tolerate JAKi.
Sales of drugs for the treatment of myelofibrosis is in the eight major markets (US, 5EU, Japan and China) amounted to USD 2,3 billion in 2021 and is projected to grow to USD 2,9 billion by 2031 (Global Data Report March 2023 – Myelofibrosis – Eight Market Drug Forecast and Market Analysis 2021-2031).
Myelofibrosis: Need for disease-modifying treatment

Source: GlobalData March 2023, 8 major markets (US, EU5, Japan and China). Presented data are based on 2031 forecast numbers.
Tasquinimod in myelofibrosis
Preclinical studies have shown that tasquinimod reduces myeloproliferation, splenomegaly (enlarged spleen), and fibrosis in models of myelofibrosis (Leimkühler et al. Cell Stem Cell. 2021). Preclinical experiments using malignant cells from patients have further shown that tasquinimod works synergistically with a JAK- or BET inhibitor to reduce spleen size and prolong survival (Fiskus et al. Blood 2023, Fiskus et al. Blood 2024). These promising results suggest that tasquinimod could be a valuable addition to the treatment options for myelofibrosis patients. In collaboration with research groups at Erasmus MC, the Netherlands and at The University of Texas MD Anderson Cancer Center, US, Active Biotech will explore myelofibrosis as a new high value orphan indication for tasquinimod within blood cancers. In February 2022, a global patent license agreement was signed with Oncode Institute, acting on behalf of Erasmus MC, for tasquinimod in myelofibrosis. Under the agreement, Oncode Institute grants to Active Biotech a global exclusive license to develop and commercialize tasquinimod in myelofibrosis. Proof of-concept studies with tasquinimod in myelofibrosis patients are ongoing in Europe and at MD Anderson Cancer Center, TX.
The study in Europe is conducted by the HOVON (Stichting HematoOncologie voor Volwassenen Nederland) research network at clinics in The Netherlands and Germany. The study is mainly funded by Oncode Institute. Preclinical results from a collaboration with a research group at MD Anderson were presented in December 2023 at an oral session and 2024 as a poster at the annual meeting of the American Society of Hematology (ASH) in San Diego, USA. The results demonstrated tasquinimod’s efficacy as monotherapy and in combination with approved and investigational drugs in models of advanced myelofibrosis. These positive results create a rationale for the ongoing clinical study in patients with myelofibrosis at MD Anderson.
Tasquinimod was granted orphan designation in myelofibrosis by the US Food and Drug Administration (FDA) in May 2022.
Ongoing clinical development in myelofibrosis
Proof-of-concept studies with tasquinimod in myelofibrosis patients are ongoing in US and Europe..
- The study in US, performed at MD Anderson Cancer Center, TX, is currently ongoing. More information about the study can be found in the box below.
- The study in Europe, conducted by the HOVON (Stichting Hemato-Oncologie voor Volwassenen Nederland) research network at clinics in The Netherlands and Germany is ongoing. The study is funded by Oncode Institute.
Tasquinimod was granted orphan designation in myelofibrosis by the FDA in May 2022.
More information can be found in the box below and at www.clinicaltrials.gov
Multiple myeloma
Multiple myeloma is an incurable blood cancer where abnormal plasma cells in the bone marrow grow uncontrollably while other blood forming cells, such as white and red blood cells and blood platelets, are suppressed. This leads to anemia, infections, destruction of bone tissue and progressive loss of renal function.
Despite new treatments which have greatly improved survival of multiple myeloma patients, the biological heterogeneity of the disease and the emergence of drug resistance is a major challenge, and the medical need of innovative treatment modalities remains high.
The market for treatment of multiple myeloma
The number of diagnosed prevalent multiple myeloma cases in the eight major markets (US, 5EU, Japan and China) in 2022 amounted to approximately 317,000 and is projected to grow to approximately 352,000 by 2032. In 2022 the US represented 49 percent of the diagnosed cases, the 5 major EU markets 26 percent and Japan and China combined 25 percent. (Global Data Report July 2024, Multiple Myeloma – Eight Market Drug Forecast 2022 – 2032).
The global sales of drugs for the treatment of multiple myeloma in the eight major markets amounted to USD 21.2 billion in 2022 and is projected to reach USD 29.3 billion in 2032. (Global Data Report July 2024, Multiple Myeloma – Eight Market Drug Forecast 2022-2032).
The market for drugs used in the treatment of multiple myeloma experiences strong growth and is expected to continue to grow strongly due to the greater incidence in an elderly population, longer progression-free and overall survival, and thanks to new treatments and combination are made available. Of the projected total market sales 2032, the US market represents around 68 percent, the five major EU markets approximately 20 percent and Japan and China for 4 and 8 percent respectively. (Global Data Report July 2024, Multiple Myeloma – Eight Market Drug Forecast 2022 – 2032).
Multiple myeloma – Market Driven by Novel Treatments
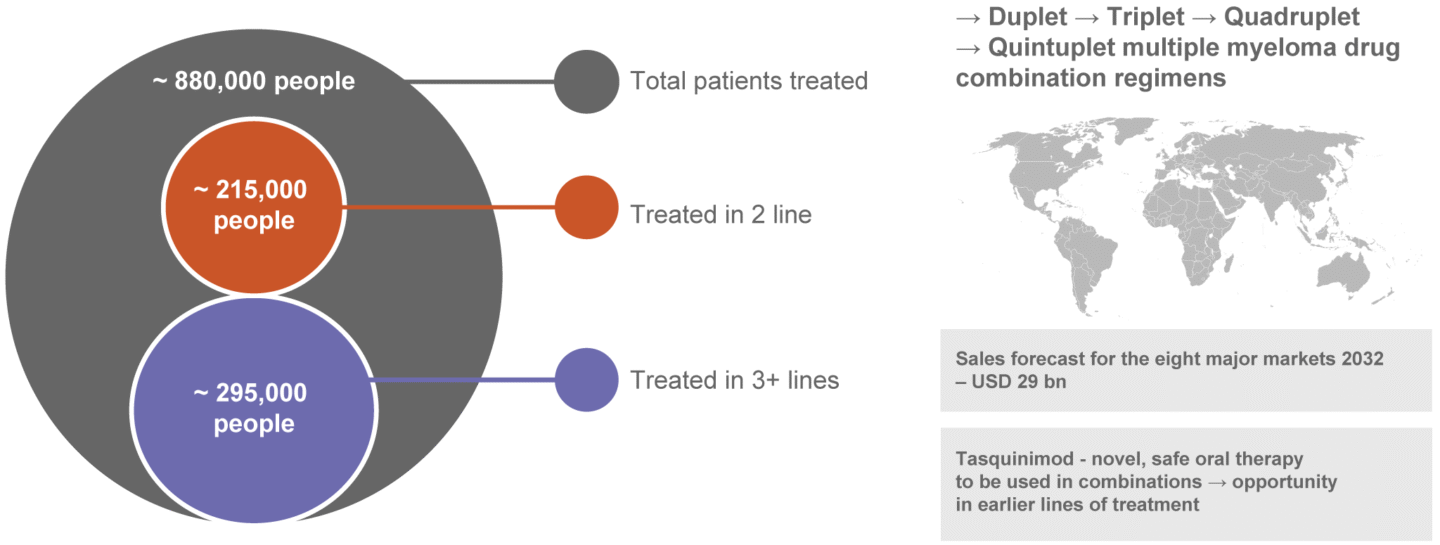
Source: Global Data Report July 2024, Multiple Myeloma – Eight Market Drug Forecast 2022-2032.
Current treatments
Multiple myeloma patients undergo several lines of treatment. In both early and later treatment lines, the goal is to reduce tumor burden, improve symptoms and thereby achieve as long a period of effective disease control as possible. To support deeper and durable responses and overcome treatment resistance patients are as standard treated with combinations of drugs from available product classes. Currently, the market is dominated by drugs that can be divided into the following classes: immunomodulatory imides (IMiDs), proteasome inhibitors (PI), monoclonal antibodies, bispecific antibodies, Chimeric Antigen Receptor T- cells (CAR-T) and alkylating agents.
Disease course of multiple myeloma

Tasquinimod in multiple myeloma
Tasquinimod is being developed as a new product class with a distinct and novel mechanism of action and thus has the potential to overcome the problem of drug resistance. The clinical safety profile of tasquinimod is well known from previous clinical phase I-III trials. Given the good tolerability and the possibility to combine with available product classes, tasquinimod has the potential to expand over time from an initial position as the 3rd line treatment to earlier lines of treatment. There is a significant market opportunity for a novel drug in a new product class in multiple myeloma.
Tasquinimod was granted orphan designation in multiple myeloma by the US Food and Drug Administration (FDA) in 2017.
Ongoing clinical development
Based on preclinical data and the previous clinical experience with tasquinimod, a clinical study was initiated, and the first patient was dosed in August 2020. The study has recruited patients with relapsed refractory multiple myeloma who have undergone at least one treatment for myeloma and has been conducted in two parts:
- First part (A) studying tasquinimod as a monotherapy
- Second part (B) studying the combination of tasquinimod and an oral standard anti-myeloma regimen (IRd; ixazomib, lenalidomide, dexamethasone)
The recruitment is completed and the data are being analysed.
More information about the ongoing study can be found in the box below . and at www.clinicaltrials.gov
Previous clinical experience of tasquinimod
Tasquinimod has been in development for the treatment of prostate cancer and has completed a phase I-III clinical development program. While the results from the phase III trial in prostate cancer showed that tasquinimod prolonged progression-free survival (PFS) compared to placebo, tasquinimod did not extend overall survival (OS) in this patient population and the development for prostate cancer was discontinued. Tasquinimod was studied in both healthy subjects and cancer patients.
Clinical effects and a favorable safety profile have been demonstrated in more than 1,500 patients, equivalent to more than 650 patient-years of exposure to tasquinimod. Extensive datasets including a regulatory package of preclinical and clinical safety and full commercial scale CMC documentation has been generated.
